How to Find Lowest Osmotic Pressure
But if normal human blood were on the right side of the membrane the osmotic pressure would be about seven atmospheres. K2FeCN6 004 4 016.

Among The Following The Solution Which Shows The Lowest Osmotic Pressure Is A 0 14 M Nacl B Youtube
Where π is the osmotic pressure.

. 3 Which leads to this van t Hoff factor. Mol-1K-1 300 K π 4926 atm. 2 The actual concentration of all species in solution H Ac-and HAc is this.
Calculating the osmotic pressure formula chemistry is done using π iMRT. 0015525 0015 1035. The confusion that often exists between osmolality and osmolarity is discussed and the clinical importance of careful distinction between these terms is emphasized.
T is the temperature. The osmotic pressure II of an ideal solution can be approximated by the Morse equation. Molar mass of glucose 72 12 96.
Osmotic weight is a significant factor influencing cells. How to Calculate Osmotic Pressure. RT is same for all.
CaCl2 005 3 015. The clinical effects of abnormal osmotic strength of parenteral solutions methods of determining osmolality and osmolarity and the p. Determining the van t Hoff factor.
R is the universal gas constant. HOPES U LIKE IT IT HELPS U. Mass of glucose 541 grams.
If the concentration of solutes on both sides of the membrane is equal then there is no tendency for water to move across the membrane and no osmotic pressure. Osmoregulation is the homeostasis system of a living being to arrive at balance in osmotic weight. The osmotic pressure of a potassium chloride solution at 300K is 50 atmospheres.
APPLYING A OSMOTIC PRESSURE CALCULATOR. NaCl 0010 2 0020 002. Sodium chloride in the solution is 1mol per 1litre.
A low serum albumin indicates. So C 1 M. Mass of glucose 0301 mol x 180 g1 mol.
M is the molar concentration of dissolved species units of molL. This illustrates how potent the influence of osmotic pressure is for membrane transport in living organisms. The molar concentration of table salt ie.
Molar mass of glucose 180 gmol. If pure water were on both sides of the membrane the osmotic pressure difference would be zero. A decrease in oncotic pressure due to a low albumin level allows fluid to leak out from the interstitial spaces into the peritoneal cavity producing ascites.
Therefore the osmotic pressure of the solution is. Albumin is essential for maintaining the oncotic pressure in the vascular system. Tonicity is the measure of this pressure.
541 grams per liter of glucose should be used for an intravenous solution to match the 765 atm at 37 degrees Celsius osmotic pressure of blood. The higher the concentration M or the temperature T of a solution the higher the osmotic pressure. Osmotic pressure the minimum pressure that must be applied to a solution to prevent the solvent of a less concentrated solution or at least the pure solvent placed beyond a semipermeable membrane from diffusing into the solution through the membrane.
R is the ideal gas constant 008206 L atm mol-1 K-1 or other values depending on the pressure units. 010 M CaBr2 B. Osmotic pressure icRT.
CeMgCl2 has the highest osmotic pressure because it breaks up into three ions in water as opposed to all the others which only break up into two. Which of the following solutions has the lowest osmotic pressure. Chemistry questions and answers.
The osmotic pressure of the 1M salt solution is 4926 atmospheres at a temperature of 27 o C. We can see from this equation that the amount of solute present in the solution will directly affect the osmotic pressure of the system. Hypertonicity is the nearness of an answer that makes cells shrivel.
015 M Ba NO22. To calculate osmotic pressure use the following formula. This video explains the concept of osmosis as well as how to obtain the osmotic pressure of a solutionSupport us.
Calculate the osmotic pressure of this solution. Therefore NaCl shows the lowest osmotic pressure. 1 Based on the 35 we have the following.
π 2 1 molL-1 00821 atmL. We need to know the molar concentration of dissolved species in order to calculate the osmotic pressure of an aqueous solution. Osmotic pressure is a colligative substance property because it depends on the concentration of the solute but not its chemical nature.
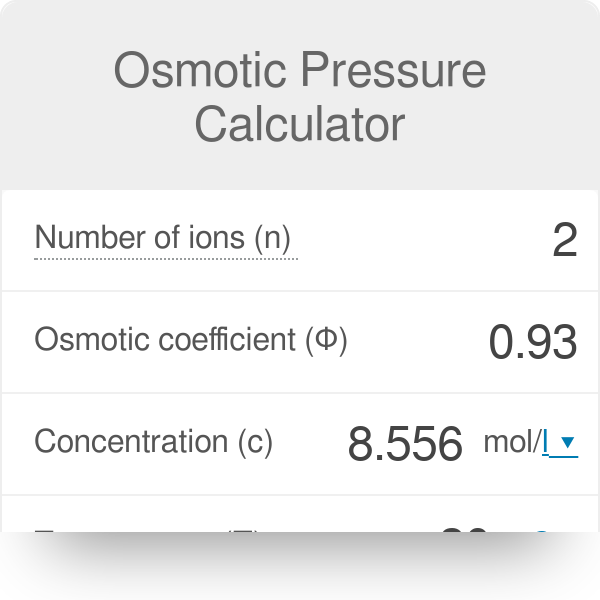
Osmotic weight estimation might be utilized for the assurance of sub-atomic loads. LatexPi i M R Tlatex Here i is the van t Hoff factor M is the molarity of the solution R is the gas constant and T is the absolute temperature in Kelvin. The equation for osmotic pressure is piiMRT.
Follow edited Dec 10 2018 at 1418. H Ac- 0015 0035 0000525 M. Click to see full answer Consequently how does albumin maintain oncotic pressure.
PLEASE MARK ME AS A BRAINLIEST. We calculate the osmotic pressure pi using the following equation. Calculate the osmotic pressure for a 010 M CaCl2 calcium chloride solution at 50 C.
I is the vant Hoff factor. Osmotic pressure is the pressure of a solution against a semipermeable membrane to prevent water from flowing inward across the membrane. 0000525 0000525 0015 - 0000525 0015525 M.
Osmotic Pressure Vocabulary. FeCl3 003 4 012. Which of the following solutions has the lowest osmotic pressure.
So value of ic are. C is the molar concentration of the solute in the solution. The Osmotic Pressure calculation example is given with a brief description below.
Working solution on how to find osmotic pressure.

Osmotic Pressure Example Youtube

Osmotic Pressure Definition Formula Video Lesson Transcript Study Com
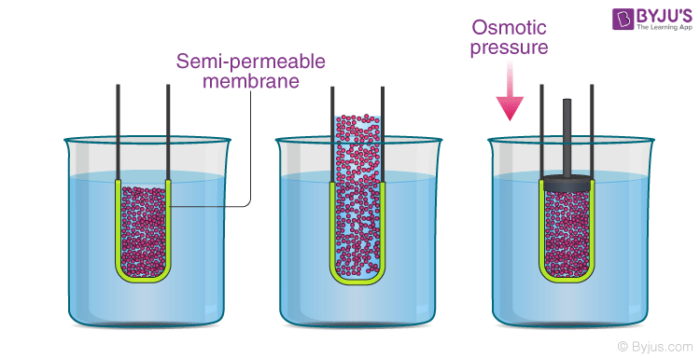
Osmotic Pressure Definition Formula Examples Solved Exercises

Osmotic Pressure And Concentration G L Of Draw Solution Download Scientific Diagram

Osmotic Pressure And Osmotic Potential Osmotic Pressure Pressure Molecules
1 Osmotic Pressure Of Single Salt Solutions As Related To The Download Scientific Diagram

Osmotic Pressure Of Salt Solutions Download Scientific Diagram

Osmotic Pressure Definition Formula Video Lesson Transcript Study Com
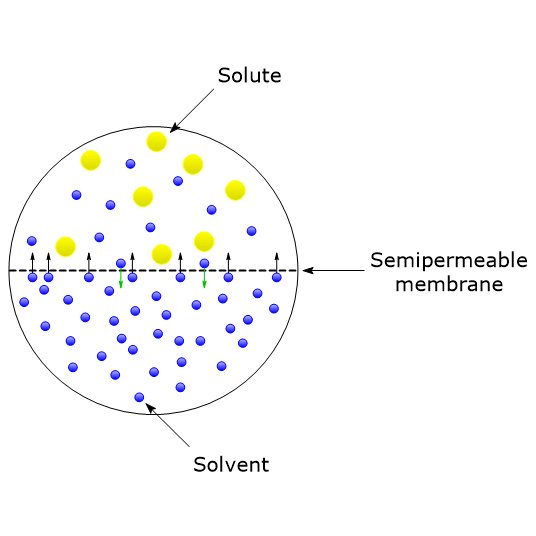
Osmotic Pressure Definition Formula And Importance
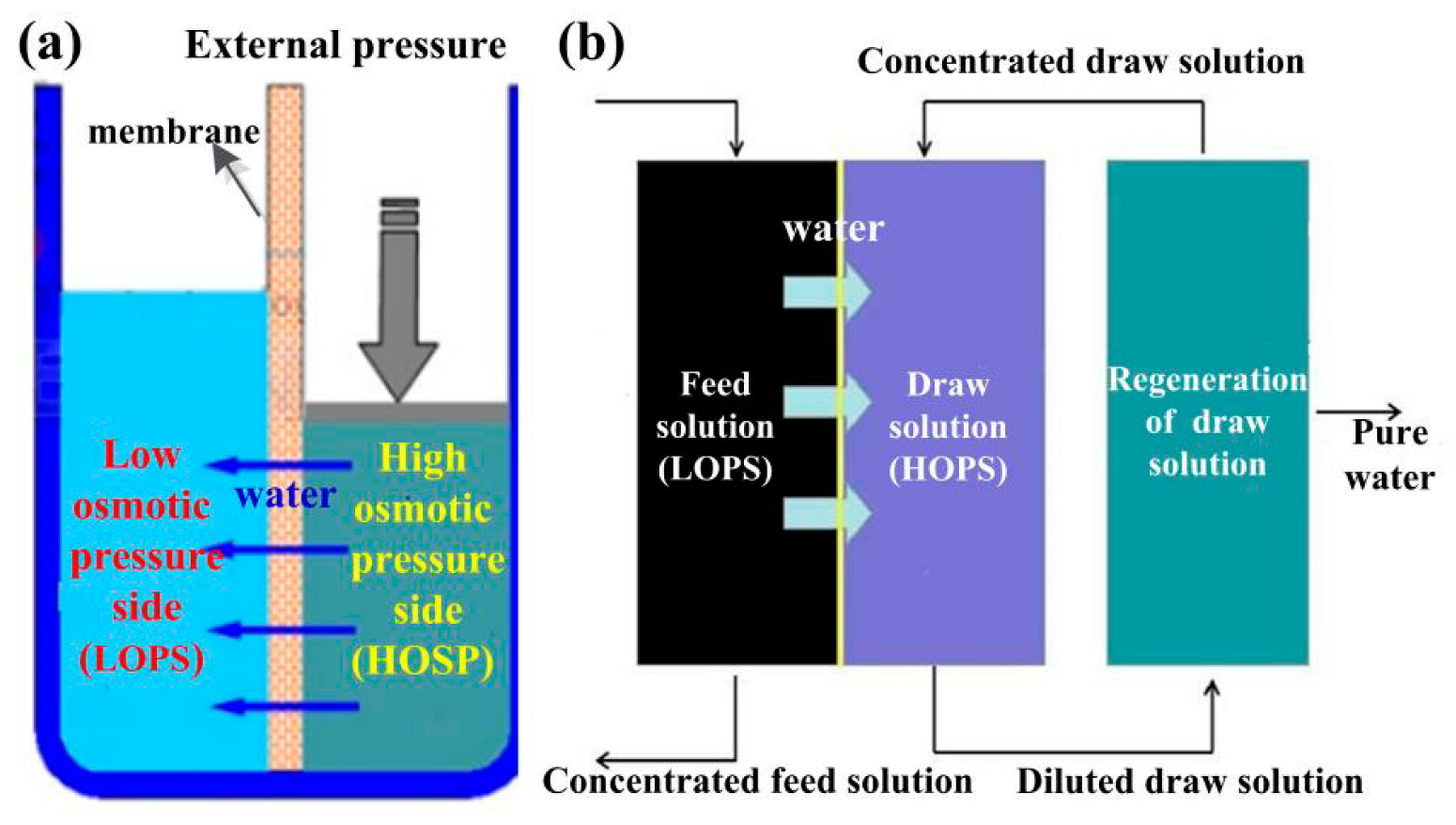
Water Free Full Text Recent Developments And Future Challenges Of Hydrogels As Draw Solutes In Forward Osmosis Process Html
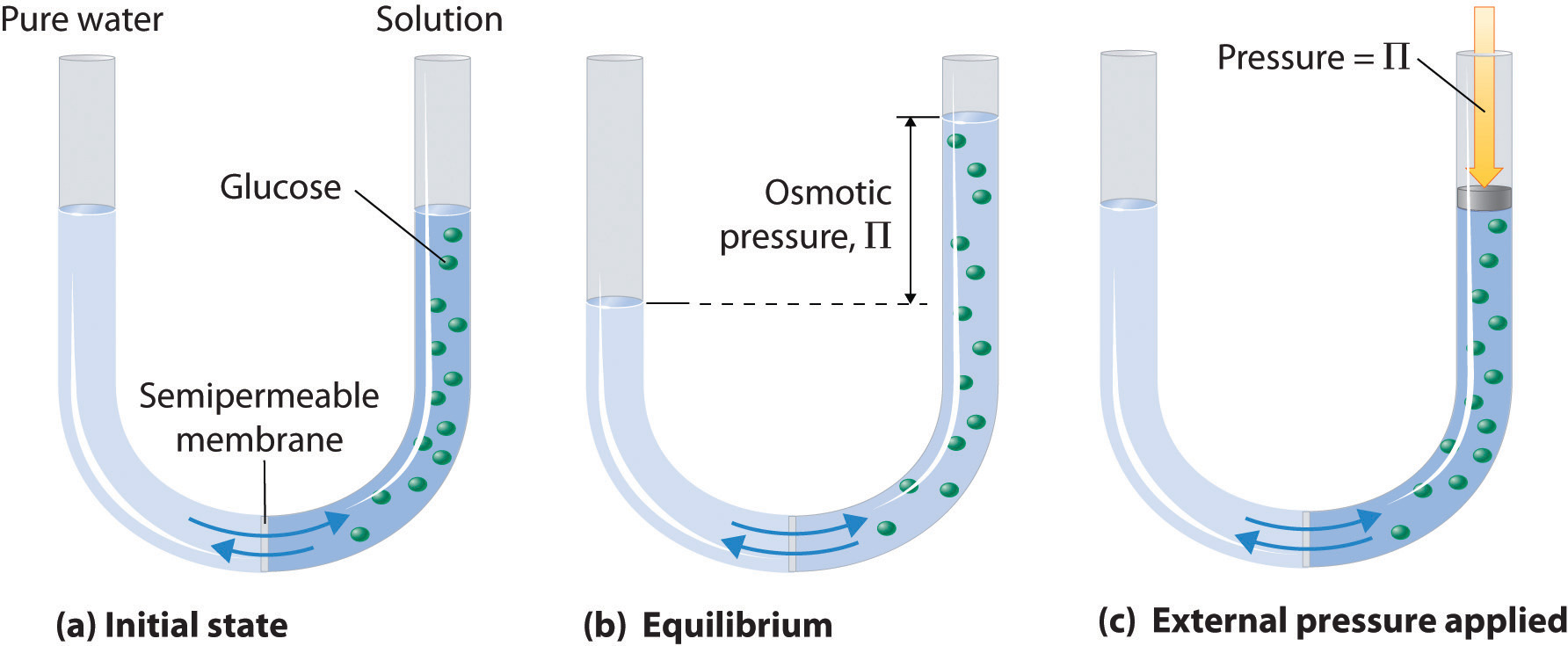
What Is The Osmotic Pressure Of A 0 050 M Solution Of Alcl 3 In Water That Is At 0 00 C Socratic

Osmotic Pressure As A Function Of Solution Concentration At 25 C For Download Scientific Diagram

Osmotic Pressure Of Nacl Solution At Different Temperatures And Download Scientific Diagram

Cells 7 Osmotic Pressure Physics And Mathematics Cell Membrane

634 Osmosis Illustrations Clip Art Istock

1 Relationship Between Concentration And Osmotic Pressure Of Solution Download Scientific Diagram

Calculate Osmotic Pressure Of A Solutions Having 0 1m Nacl 0 2m Na 2 So 4 And 0 5m Youtube

Comments
Post a Comment